Altered Corticobrainstem Connectivity during Spontaneous Fluctuations in Pain Intensity in Painful Trigeminal Neuropathy
A new publication led by Luke Henderson and Noemi Meylakh at NeuRA Imaging reveals cortical systems underlying changes in perceived pain in painful trigeminal neuropathy.
Pain perception is vital to survival, warning us of danger and motivating us to remove ourselves from it. It is adaptive and transient. However, in some cases, pain persists beyond the period of normal healing, becoming maladaptive and chronic.
Neuropathic pain, chronic pain resulting from damage to the nervous system, can occur in the absence of any external stimulus. Individuals with chronic neuropathic pain can undergo daily and even moment-to-moment fluctuations in ongoing pain intensity.
Previous work has demonstrated that signal coupling between brainstem regions including the midbrain periaqueductal gray (PAG) matter, rostral ventromedial medulla (RVM), and spinal trigeminal nucleus (SpV) was associated with perceived spontaneous pain intensity fluctuations in painful trigeminal neuropathy (PTN) patients (Mills et al., 2020).
In this paper, we were interested in determining the cortical inputs that first contact this brainstem circuitry to drive these effects.
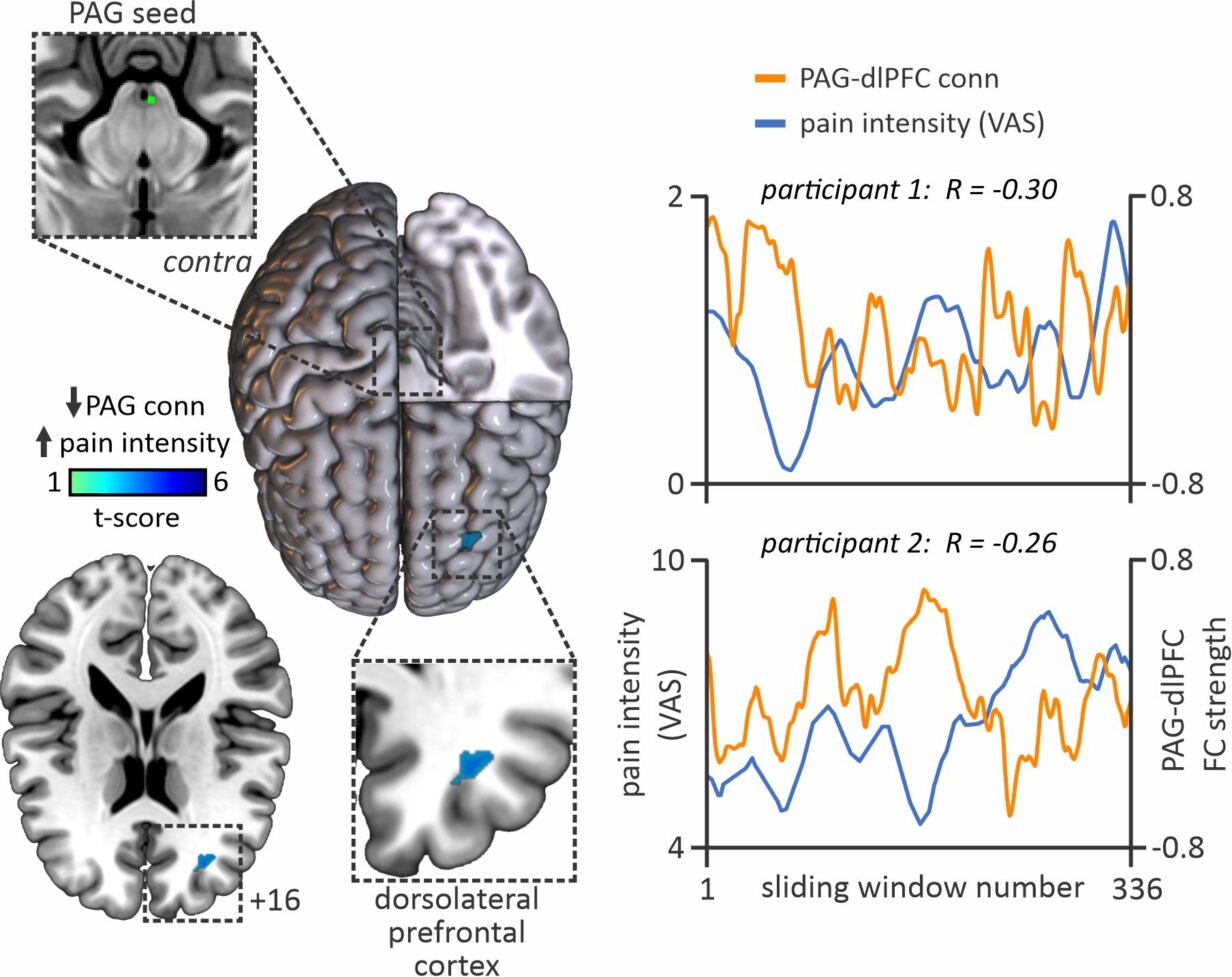
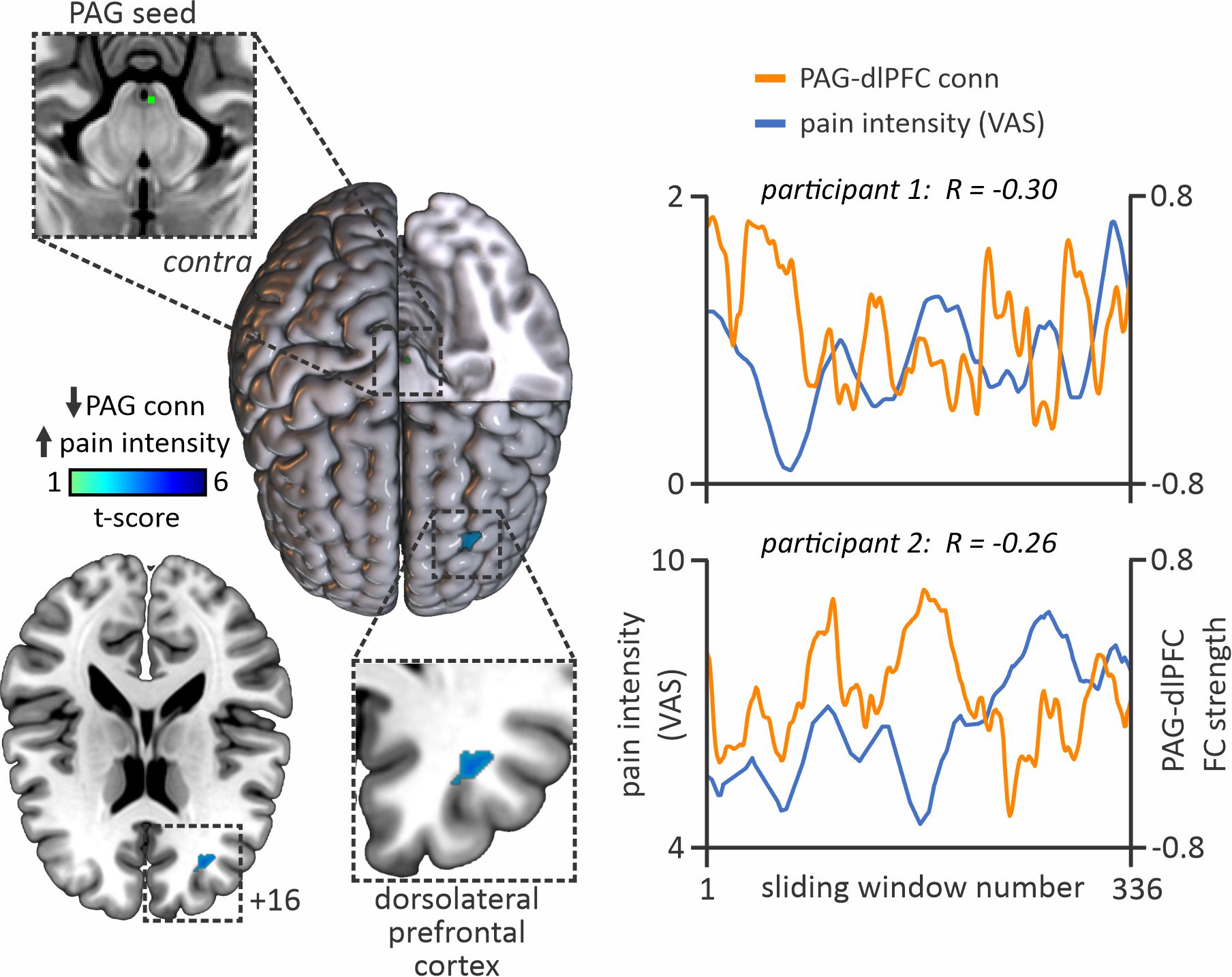
Twenty-four PTN participants had a 12-minute functional magnetic resonance imaging (fMRI) scan conducted, throughout which they were instructed to rate their ongoing pain experience using a computerised Visual Analogue Scale (CoVAS, Medoc) in real-time. Inspection of pain intensity ratings across the 12 min of fMRI scanning, revealed that in some individuals, pain fluctuated greatly (n=13), while in others, pain remained relatively stable (n=11).
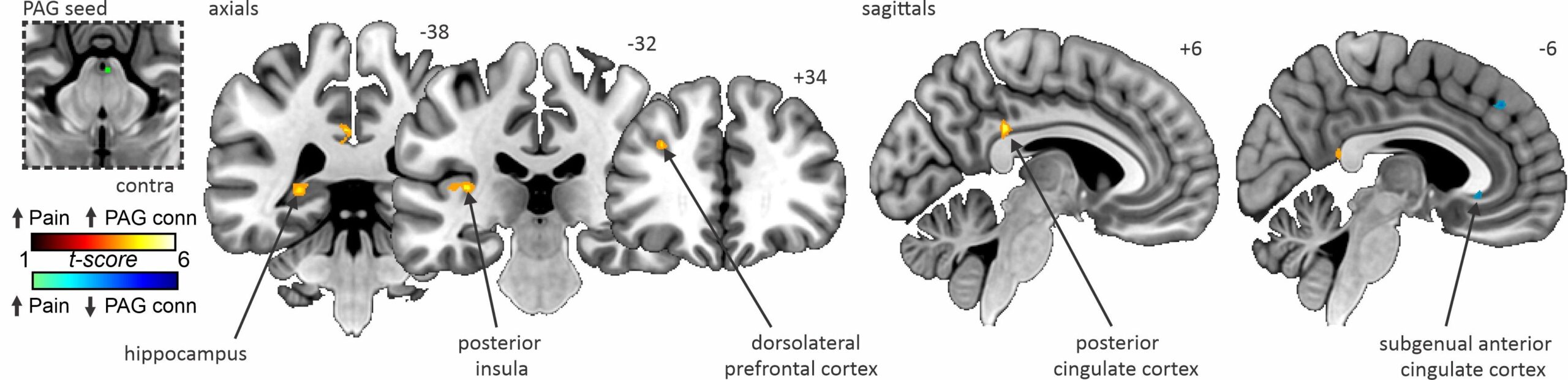
The results of this study demonstrated that fluctuations in ongoing pain intensity are associated with altered communication between cortical areas associated with pain processing and modulation, and the brainstem pain-modulatory circuitry. When pain was high, we observed higher PAG coupling with the hippocampus, posterior insula, dorsolateral prefrontal cortex (dlPFC) and posterior cingulate cortex compared with periods where pain was low.
Conversely, when pain was low, we found greater PAG coupling with the subgenual anterior cingulate cortex compared with when the pain was high. There were no overall changes in PAG coupling within these sites in PTN participants when pain remained stable.
Furthermore, PAG-dlPFC coupling was negatively associated with ongoing pain intensity ratings, i.e. the higher the pain, the lower the PAG-dlPFC coupling and vice versa.
These data show that moment-to-moment changes in cortical communication with brainstem pain-modulatory circuits residing in the midbrain PAG likely contribute to fluctuations in spontaneous pain intensity in individuals suffering chronic neuropathic pain.
- Mills EP, Alshelh Z, Kosanovic D, Di Pietro F, Vickers ER, Macey PM, Henderson LA (2020) Altered brainstem pain-modulation circuitry connectivity during spontaneous pain intensity fluctuations. J Pain Res 13:2223–2235. 10.2147/JPR.S252594
Recent Comments