Ethanol reduces brain electrical tissue conductivity in social drinkers in a region-specific pattern
A new publication led by Lindy Rae and Jun Cao at NeuRA Imaging shines light on the effect of alcohol on brain conductivity in social drinkers.
Ethanol has two main effects on the brain. It acts as a stimulant at very low concentrations but is also a sedative, substantially reducing brain metabolism, particularly through activity at inhibitory GABA(A) receptors. Here, based on the hypothesis that tissue conductivity reflects brain metabolic activity, brain tissue electrical conductivity was measured using magnetic resonance electrical properties tomography (MREPT) in 52 healthy young participants both before and after the consumption of alcohol.
As alcohol is known to decrease brain activity, we aimed to test whether tissue electrical conductivity changes would reflect this decrease. If this were to be the case, this would improve our understanding of the biophysics of tissue electrical conductivity as well as open the prospect of using non-invasive measures of electrical conductivity as a surrogate marker for brain activity in health and disease.
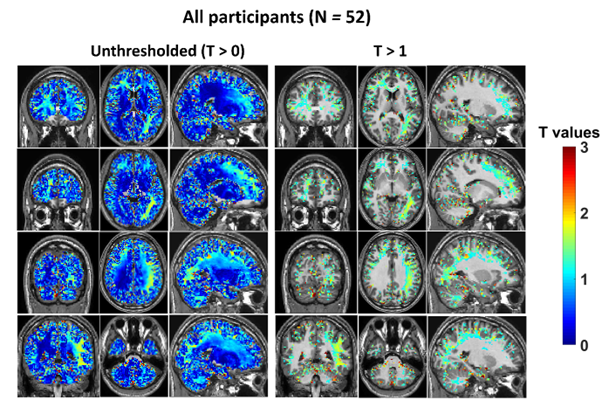
Whole group (N = 52) analysis showed areas of decreased conductivity which did not survive statistical thresholding.

Given the known heterogeneity in brain response to alcohol three groups were generated: those with strong conductivity decrease ( > 0.1 S/m; N = 33); weak conductivity increase (0.02 S/m < < 0.1 S/m; N = 9) and those showing no significant response ( < 0.02 S/m, N = 10).
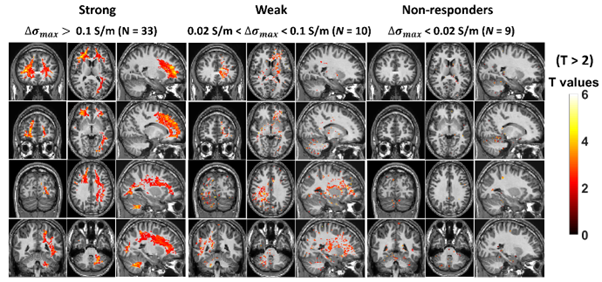
A spatially consistent pattern of significantly decreased conductivity was seen in those with a strong response with frontal and occipital white matter and cerebellum particularly impacted, while a similar pattern was observed in the weak response group. There was no relationship with breath alcohol levels, alcohol use, age, ethnicity or sex. The patterns of response in the strong responders were different depending on whether their alcohol levels were ascending or descending during the scan.
Observations from PET FDG uptake studies have reported considerable heterogeneity in the response to ethanol, particularly at low concentrations. The spatial pattern of decreased conductivity is highly similar to that reported in FDG PET studies with decreases in the frontal cortex and cerebellum being the most commonly reported findings.
A previous relationship has been reported between FDG uptake and reported (from autopsy) density of benzodiazepine receptors (α1, α2, α3 or α5-containing GABA(A) receptors; (1) but the cause(s) of decreased conductivity remain to be determined. Certainly ethanol, a known sedative, is known to greatly decrease metabolic activity on acute administration (2) and it likely does this through interaction with receptors.
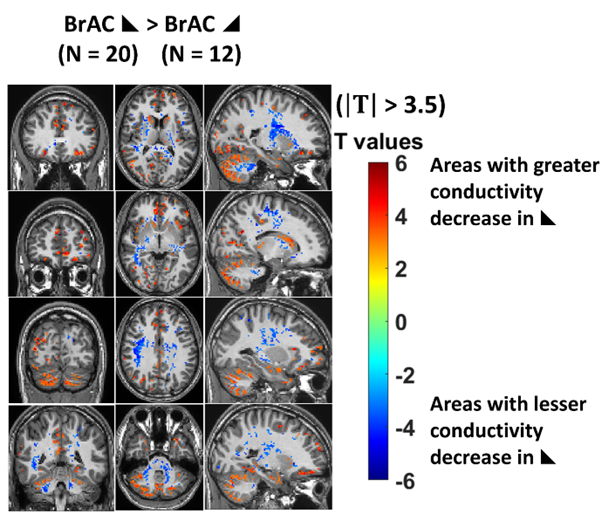
Differences in physiological, behavioural and cognitive performance have been reported depending on whether alcohol levels were rising or falling (3-7) which suggests that different areas of the brain are affected by the direction of alcohol concentration change. The gray matter in the cerebellum was particularly impacted by falling alcohol levels (Fig. 5) with a greater decrease in conductivity under falling conditions among the strong responders.
Cerebellar motor and cognitive functions are reported to recover impairment to drug-free levels under falling conditions, but errors caused by alcohol fail to recover (8). This is in line with the role of the cerebellum as a predictor-corrector (9) and highlights the role of the time course of alcohol intoxication in understanding human response to alcohol.
These results, reported in JMRI reflect the effect of alcohol on underlying brain activity. Tissue conductivity may therefore provide a useful marker of brain activity for clinical, pharmacological and research studies.
- Volkow ND, Hitzemann R, Wolf AP, et al. Acute effects of ethanol on regional brain glucose metabolism and transport Psychiatry Research-Neuroimaging 1990;35(1):39-48.
- Rae CD, Davidson JE, Maher AD, et al. Ethanol, not detectably metabolized in brain, significantly reduces brain metabolism, probably via action at specific GABA(A) receptors and has measureable metabolic effects at very low concentrations. Journal of Neurochemistry 2014; 129: 304-314.
- Nicholson ME, Wang M, Airhihenbuwa CO, Mahoney BS, Christina R, Maney DW. Variability in behavioral impairment involved in the rising and falling BAC curve. J Stud Alcohol 1992;53(4):349-356.
- Hendler RA, Ramchandani VA, Gilman J, Hommer DW. Stimulant and sedative effects of alcohol. Behavioral neurobiology of alcohol addiction 2013:489-509.
- Giancola PR, Zeichner A. The biphasic effects of alcohol on human physical aggression. J Abnorm Psychol 1997;106(4):598.
- Schweizer TA, Vogel-Sprott M, Danckert J, Roy EA, Skakum A, Broderick CE. Neuropsychological profile of acute alcohol intoxication during ascending and descending blood alcohol concentrations. Neuropsychopharmacology 2006;31(6):1301-1309.
- Pihl RO, Paylan SS, Gentes‐Hawn A, Hoaken PN. Alcohol affects executive cognitive functioning differentially on the ascending versus descending limb of the blood alcohol concentration curve. Alcoholism: Clinical and Experimental Research 2003;27(5):773-779.
- Schweizer TA, Vogel-Sprott M. Alcohol-impaired speed and accuracy of cognitive functions: a review of acute tolerance and recovery of cognitive performance. Exp Clin Psychopharmacol 2008;16(3):240.
- Sokolov AA, Miall RC, Ivry RB. The cerebellum: adaptive prediction for movement and cognition. Trends in cognitive sciences 2017;21(5):313-332.
Recent Comments