PUBLICATION SPOTLIGHT New Atlas Charts Structural Variations in the Human Tongue
A new publication led by Rob Lloyd and Lynne Bilston at NeuRA Imaging identifies gendered differences in the structure of the tongue, and provides a structural atlas for wider use.
The complex structure of the tongue provides a large range of motion, allowing it to form the different shapes needed to manipulate food while chewing and swallowing, during speech and while breathing.
Differences in the shape of the facial bones (i.e. mandible and hyoid) will change the lines of action of the tongue’s muscles, and how it deforms during everyday tasks. Detailed measurements of the tongues structure are needed to accurately simulate tongue motion.

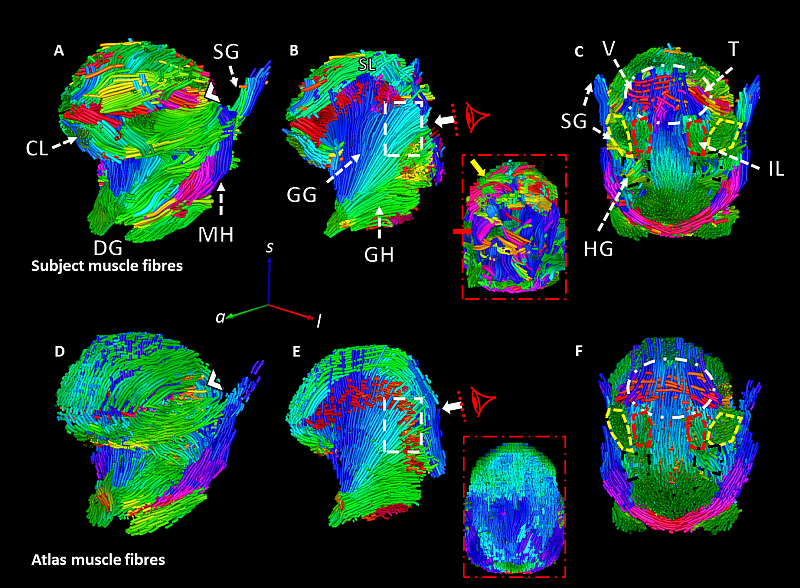
Fig. 1. Estimated muscle tracts for a single representative subject (A-C) and the muscle architecture for the same subject estimated from the filtered muscle tracts of the second fold’s atlas (D-F). The white arrow in B indicates a region of the superior longitudinal (SL) that was unable to be reconstructed for this subject due to poor quality image data but is plausibly reconstructed from the atlas refit to the subject’s anatomy. Similarly, the dashed rectangle in B indicates where the transverse muscles (T) crossing the genioglossus (GG) were unable to be reconstructed for this subject from the raw data but were plausibly estimated from the atlas (E). The dashed oval (C&F) indicates the boundary where the crossing tracts of the T, vertical (V) and SL could not be estimated. The chevron (〈) in (A) Indicates where palatoglossus (PG) merges with the transverse muscle tracts. Longitudinal muscles are highlighted in the coronal views (C &F) where the styloglossus (SG) is outlined in yellow, the hyoglossus (HG) in black, and the inferior longitudinal muscles are in red. GH: geniohyoid, MH: mylohyoid, and CL: combined longitudinal. Directions of fibre tracts are colour-coded by orientation (AP = green, SI = blue, LR = red). Taken from Lloyd et al. (2025).
Existing models of the tongue have been approximated from dissections of cadaveric tongues, which have provided a general description of the tongues structure but fail to account for the influence of the muscle attachment sites. Recently, diffusion weighted imaging (DWI) has been used to collect in vivo measurements of muscle structure (Voskuilen et al., 2018). However, low image signal and noise from physiological motion resulted poor estimates of muscle tracts, that limit the motion of simulated tongues (Kappert et al., 2021).
In this paper we aimed to build an atlas of the tongue, that could be used with computational models to simulate tongue function, or supplement noisy diffusion images.
Twenty healthy participants had mDIXON and DWI of the oral cavity collected. Constrained spherical deconvolution was used to estimate the muscle fibre directions and account for interdigitated muscles like the transverse and vertical muscles within the tongues body. Multi-channel registration was used to align all participant data and calculate the population average.
The atlas was able to accurately estimate the tongue structure of individual subjects, and improve the estimation of muscle tracts of the tongue (Fig. 1). We used the atlas to build a statistical shape model to characterise the variances in tongue structure. The largest source of variation was associated with elevation of the hyoid bone (Fig. 2). Showing that on average that males had a lower hyoid bone, and larger suprahyoid muscles.
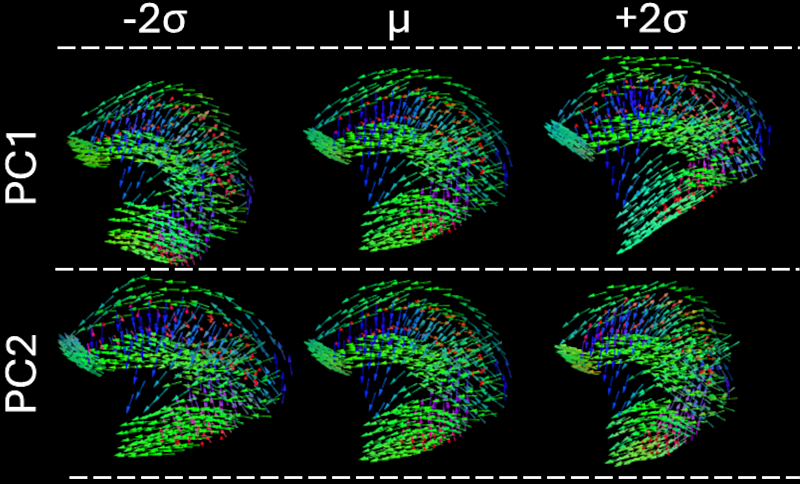
Fig. 2. Visualisation of the variation in tongue shape within the sampled population as described by principal components 1 to 2 between ±2 standard deviations from the atlas tongue. All panels show a midsagittal cross-sectional view of the tongue. Directions of fibre tracts are colour-coded by orientation (AP = green, SI = blue, LR = red). Edited from Lloyd et al. (2025).
The differences in the orientation of the tongue’s muscles will alter the combination of muscles recruited to achieve the same motion in people with different facial features.
A detailed discussion of these findings has been reported in Computers in Biology and Medicine – https://doi.org/10.1016/j.compbiomed.2025.110006
We have also made the tongue atlas freely available for public use at https://doi.org/10.17605/OSF.IO/Z7U59.
- Voskuilen, L., Mazzoli, V., Oudeman, J., Balm, A.J., van der Heijden, F., Froeling, M., de Win, M.M., Strijkers, G.J., Smeele, L.E., Nederveen, A.J., (2019). Crossing Muscle Fibers of the Human Tongue Resolved in Vivo Using Constrained Spherical Deconvolution. Journal of Magnetic Resonance Imaging 50, 96-105. https://doi.org/10.1002/jmri.26609
- Kappert, K.D.R., Voskuilen, L., Smeele, L.E., Balm, A.J.M., Jasperse, B., Nederveen, A.J., van der Heijden, F., (2021). Personalized Biomechanical Tongue Models Based on Diffusion-Weighted MRI and Validated Using Optical Tracking of Range of Motion. Biomechanics and Modeling in Mechanobiology 20, 1101-1113. https://doi.org/10.1007/s10237-021-01435-7n
- Lloyd R.A., Dissanayake E, Jugé L., Bolsterlee B., Ball I.K., Bilston L.E. (2025). How mandibular and hyoid morphology alters tongue muscle architecture in healthy adults: An anatomical atlas and statistical shape model of the tongue. Computers in Biology and Medicine 189:110006. https://doi.org/10.1016/j.compbiomed.2025.110006
- Lloyd R.A., Dissanayake E., Jugé L., Bolsterlee B., Ball I.K., Bilston L.E. (2025). Human tongue muscle atlas. Open Science Framework. https://doi.org/10.17605/OSF.IO/Z7U59
Recent Comments